|
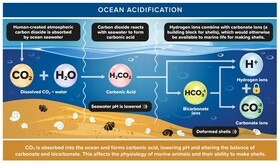
Ocean acidification is harmful for a multitude of reasons. For one, higher acidity has been found to cause a decrease in metabolism and immune response in marine life and interfere with echolocation (You may want to explain echolocation here). Ocean acidification also affects the shells of marine animals because of a compound called calcium carbonate. The carbonate formed from the combination of carbon dioxide and water can combine with calcium to form calcium carbonate, which is the main compound found in the shells and skeletons of marine life. It is also important to note that the dissociation of carbonic acid to form carbonate is a reversible process. The more acidic the water is, the more likely it is for carbonate to combine with one or two hydrogen ions to form carbonic acid or bicarbonate. As the pH decreases, so does the amount of carbonate, which causes a subsequent decrease in the amount of calcium carbonate. Without enough calcium carbonate, shells and skeletons will be weak, deformed, and prone to degradation.
By reducing carbon emissions, we can combat global warming and ocean acidification simultaneously. Everyone should care about this issue, especially youth, because it will be up to our generation to find a solution before it is too late. The most effective way to combat the problem would be a sustainable renewable energy source that can be used on a large scale. My proposal is nuclear fusion[1], the same energy that powers stars. Nuclear fusion produces no carbon dioxide and the reactant, seawater[2], is plentiful and naturally replenished. Additionally, nuclear fusion reactors produce no high activity, long-lived nuclear waste. If the right technology is developed, nuclear fusion could provide an unlimited supply of clean energy, though this is still decades away. Works Cited https://www.youtube.com/watch?v=fgBozLCGUHY https://www.youtube.com/watch?v=GL7qJYKzcsk https://www.youtube.com/watch?v=Wc8SJqAPVaM https://www.diffen.com/difference/Nuclear_Fission_vs_Nuclear_Fusion Ocean Acidification Nuclear Fusion Reactor [1] Nuclear fusion is a process where two atomic nuclei are forced together. This is not to be confused with nuclear fission, the splitting of an atom to produce energy, which was used to power atomic bombs. Fusion produces 4 times the energy of fission and is much safer and easier to control. [2] To be precise, the reactants are isotopes of hydrogen, deuterium and tritium, found in seawater. Comments are closed.
|
|